What is the Mantoux test?
The Mantoux test, also known as the tuberculin skin test, is a test used to screen for tuberculosis (TB) infection. It is not used to diagnose active TB disease. The test is performed by injecting a small amount of tuberculin purified protein derivative (PPD) into the skin of the forearm. If the person has been exposed to TB, the body will react to the PPD and a small, hard bump (induration) will form at the injection site within 2 to 3 days. The size of the induration is measured to determine whether the person has been exposed to TB.
What are the benefits of the Mantoux test?
The Mantoux test is a simple, painless, and inexpensive test that can be used to screen latent tuberculosis . It is also a very accurate test, with a sensitivity of over 90%. This means that if the test is positive, it is very likely that the person has TB.
clinical indication or utility of Mantoux test
The Mantoux test is recommended for people who are at high risk of getting TB, such as:
- People who have been in close contact with someone who has TB
- People who live or work in areas with a high incidence of TB
- People who are immunocompromised, such as people with HIV/AIDS or cancer
- To rule out latent Tuberculosis before initiating TNF alpha inhibitors drugs (Eternacept, adalimumab) that are used to treat rheumatoid arthritis (RA), ankylosing spondylitis , psoriatic arthritis , and plaque psoriasis.1
- TST is the recommended method of testing for children younger than 5 years of age. It should be noted that the American Academy of Pediatrics (AAP) recommends that either a TST or TB blood test (interferon-gamma release assay [IGRA]), can be used in children 2 years and older
contraindication :The Mantoux test is not advised only for persons who had a severe reaction to a previous Mantoux test.
Procedure of Mantoux test
Materials Needed:
- Tuberculin PPD solution
- Tuberculin syringe and needle
- Alcohol swab
- Ruler , marking pen
- Bandage or adhesive tape
- Tuberculin PPD 1 TU for less than12 yrs of age & 2 TU for more than12 yrs of age
Mantoux Reading
Patient Assessment:
Explain the procedure to the patient and address any concerns or questions.
Patient Positioning:
Have the patient sit or lie down comfortably, exposing their forearm (usually the left forearm ).
Locate the Injection Site: Identify the appropriate injection site on the forearm. It is usually placed on the inner side (Flexor ) of the forearm, about 4-6 inches below the elbow.
Injection:
Disinfect the site of injection with a spirit swab and allow it to dry.
Draw 0.1 ml of PPD by using tuberculin syringe.
Using 26 G needle, Insert the needle Intradermally at a 5-15 degree angle at the site which is already disinfected. Inject the PPD intradermally to make the deposition wheal, about 6-8 mm in
diameter which will rise up to the point of needle.
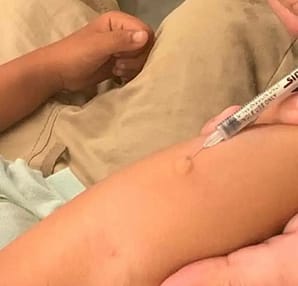
Mark the Site:
Mark the area of injection with a pen.
Read the results after 48-72 hours by measuring horizontal diameter of indurations
only in millimeters
Post-Injection Care:
Instruct the patient not to touch, scratch, or rub the injection site.
Reading the Test:
- Measure the size of the raised area or bump at the injection site using a millimeter ruler. Only measure the induration (hard, raised area), not any redness or swelling.
- Record the measurement and note the presence or absence of induration.

Interpretation
1.A tuberculin skin test (TST) result of ≥5 mm is considered positive for tuberculosis (TB) in the following conditions:
- Those who’ve had recent contact with someone who has TB
- Patients with fibrotic changes on a chest X-ray consistent with old TB
- People who are immunocompromised: This includes people with HIV/AIDS, cancer, Organ transplant recipients or other conditions that weaken the immune system
- Patients who are immunosuppressed for reasons other than organ transplant (for example, those taking the equivalent of >15 mg/day of prednisone for 1 month or longer, those taking TNF-α antagonists)
2.A tuberculin skin test (TST) result of ≥10mm is considered positive for tuberculosis (TB) in the following conditions:
People born in countries where TB disease is common, including Mexico, the Philippines, Vietnam, India, China, Haiti, and Guatemala, or other countries with high rates of TB
- People who abuse drugs
- Mycobacteriology laboratory workers
- People who live or work in high-risk Crowded areas (e.g., nursing homes, homeless shelters, or correctional facilities)
- People with certain medical conditions that place them at high risk for TB (e.g., silicosis, diabetes mellitus, severe kidney disease, certain types of cancer, and certain intestinal conditions)
- People with a low body weight (<90% of ideal body weight)
- Children younger than 5 years of age
- Infants, children, and adolescents exposed to adults in high-risk categories
3. An induration of 15 or more millimeters is considered positive in
People with no known risk factors for TB
Source: Centers for Disease Control and Prevention 2020
If the diameter of the Erythema is greater than 10 mm and induration is absent
the injection may have been made too deeply and retesting is indicated.
What are the causes of false-positive Mantoux tests?
There are a number of things that can cause a false-positive Mantoux test, including:
- Previous TB vaccination with the bacille Calmette-Guérin (BCG) vaccine
- Infection with nontuberculous mycobacteria (NTM)
- Allergies
What are the causes of false-negative Mantoux tests?
There are a number of things that can cause a false-negative Mantoux test, including:
- Early TB infection
- Weakened immune system
- Babies younger than 6 months
- Incorrect method of giving the PPD injection
- Incorrect measuring or interpretation of reaction
what is Interferon-gamma release assays or IGRAs?
interferon-gamma release assays (IGRAs) are blood tests that can be used to diagnose tuberculosis (TB) infection.
Advantages of IGRAs over the Mantoux test:
- More accurate
- Not affected by BCG vaccination
- Can be used in people who are allergic to the Mantoux test
