Introduction
Perl’s staining, also known as Prussian blue staining, is a specialized histochemical technique widely used in histopathology to detect the presence of iron in biological tissues. This method is particularly valuable in diagnosing various diseases related to iron metabolism, such as hemochromatosis and hemosiderosis. The staining highlights iron deposits, allowing pathologists to visualize and quantify iron accumulation within cells and tissues.
Perl’s staining technique was first developed by the German pathologist Max Perls in the late 19th century. His method involved the use of potassium ferrocyanide, which reacts with ferric iron (Fe3+) to produce an insoluble blue pigment, ferric ferrocyanide, also known as Prussian blue. This innovation provided a significant advancement in the ability to detect and study iron deposits in tissues, paving the way for better understanding and diagnosis of iron-related disorders.
Principle of the Technique
Perl’s staining is based on the chemical reaction between ferric iron (Fe³⁺) and potassium ferrocyanide, resulting in the formation of an insoluble blue compound known as Prussian blue (ferric ferrocyanide). The process can be summarized as follows:
- Ferric Iron Interaction: The tissue is treated with hydrochloric acid to liberate ferric ions from hemosiderin (a storage form of iron).
- Formation of Prussian Blue: The liberated ferric ions react with potassium ferrocyanide, producing an intense blue pigment indicative of iron deposits.
Here, the ferric ions react with ferrocyanide ions to form an insoluble blue compound, ferric ferrocyanide (Prussian blue), which precipitates at the site of the iron deposits.
Procedure of Perl’s staining in histopathology
preparation of acid ferrocyanide solution
| 1% Aqueous pottasium ferrocyanide | 20 ml |
| 2% Aqueous hydrochloric acid (HCL) | 20 ml |
Positive control should be used with all test sections. A known positive control tissue containing hemosiderin pigment.
- Fixation: Tissue samples are first fixed using formalin . Avoid use of acid fixatives. chromates also interfere with preservation of iron in tissues.
2. Deparaffinize and hydrate slides to distilled water. The tissue sections are deparaffinized with xylene and rehydrated through a series of alcohol washes.
3. Treat section with the freshly prepared acid ferrocyanide solution for 10-20 minutes.
4. Wash well in distilled water.
5. Lightly stain with 1% eosin or 0.1% nuclear fast red as a counter stain .
6. Wash rapidly in distilled water.
6. Dehydrate clear and mount in DPX. The stained sections are dehydrated through ascending alcohol series, cleared in xylene, and mounted with a coverslip for microscopic examination.
Result: Ferric iron – blue, Nuclei Red, Background – Pink
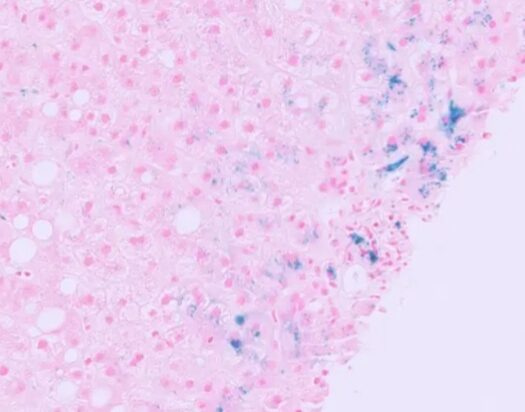
Applications
Perl’s staining is primarily used for the detection of hemosiderin, an iron-storage complex, in tissues. This technique has several key applications in medical diagnostics:
- Hemochromatosis: Perl’s staining helps in diagnosing hereditary hemochromatosis by identifying excessive iron deposits in the liver and other organs.
- Hemosiderosis: This condition, often secondary to multiple blood transfusions, results in iron overload which can be detected using Perl’s staining.
- Liver Diseases: Chronic liver diseases, such as cirrhosis and chronic hepatitis, often show iron deposition which can be visualized using this staining method.
- Sideroblastic Anemia: Perl’s stain can highlight ring sideroblasts in the bone marrow, a characteristic feature of this anemia.
- Neurological Conditions: In cases of neurodegenerative diseases, Perl’s staining can reveal iron accumulation in the brain, providing insights into disease mechanisms.
Limitations:
While Perl’s staining can indicate the presence and distribution of iron, it does not provide quantitative data on the amount of iron present.
References:
Bancroft, J. D., & Gamble, M. (Eds.). (2008). Theory and Practice of Histological Techniques (6th ed.). Elsevier Health Sciences.
Kumar, V., Abbas, A. K., Aster, J. C., & Perkins, J. A. (2021). Robbins Basic Pathology (11th ed.). Elsevier.
