Introduction:
Naegleria fowleri, commonly referred to as the “brain-eating amoeba,” is a free-living amoeba found in warm freshwater environments. Primary amoebic meningoencephalitis (PAM) is a rare disease but is associated with high mortality rates.
The infection occurs when Naegleria fowleri enters the CNS through the nasal passages, usually during activities involving freshwater exposure, such as swimming in warm bodies of freshwater. While infections caused by Naegleria fowleri are extremely rare, they are also highly lethal, with a mortality rate of over 95%.

sign and Symptoms Common early symptoms of Primary amoebic meningoencephalitis (PAM) include severe headache, fever, nausea, vomiting, and a stiff neck. As the infection progresses, neurological symptoms may develop, including seizures, altered mental status, confusion, hallucinations, and coma.
Timely and accurate laboratory diagnosis plays a crucial role in identifying this pathogen and enabling early intervention to improve patient outcomes. In this article, we delve into the various laboratory methods used for diagnosing Naegleria fowleri infections.
Specimen Requirement
The CSF is the specimen of choice for demonstration of the amebae.
Brain tissue biopsy allows for direct visualization of the amoebae and detection of specific antigens using immunohistochemistry, assisting in confirming the presence of Naegleria Fowleri within the brain.
Microscopic Examination:
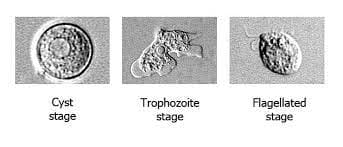
Microscopic examination of cerebrospinal fluid (CSF) is the primary diagnostic method for Naegleria fowleri. A lumbar puncture is performed to obtain a sample of CSF, which is then examined under a microscope. The characteristic morphology of Naegleria fowleri allows for its identification.
The trophozoites of Naegleria fowleri are large, ranging from 8 to 15 μm in diameter, and possess a distinctive amoeboid shape with a central nucleus and pseudopodia. The presence of these trophozoites in the CSF is a strong indicator of Naegleria fowleri infection.

Direct CSF wet examination under phase contrast microscope aids in early diagnosis
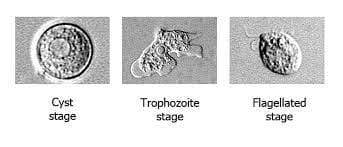
Direct wet-mount CSF microscopy for Naegleri fowleri
To directly observe the presence of Naegleria fowleri, a direct wet-mount microscopy technique can be employed using cerebrospinal fluid (CSF) samples. Here’s how it’s done:
- Centrifugation: The CSF sample is centrifuged at 150 xg for 5 minutes. This process separates the sediment from the supernatant.
- Sediment Preparation: The supernatant is carefully aspirated, leaving the sediment at the bottom of the tube. The sediment is then suspended in the remaining fluid.
- Slide Preparation: A drop of the sediment suspension is placed on a microscope slide are (mixed with 1 mL of distilled water) . A coverslip is gently mounted over the drop, creating a slide for examination.
- Microscopic Examination: The prepared slide is examined using compound light microscopy, utilizing 10X and 40X objectives. Ideally, phase contrast microscopy is used for enhanced visualization.
- Observation: Close observation is crucial as Naegleria fowleri trophozoites can be identified based on their active directional movements. The trophozoites exhibit lobopodia, which are extensions and retractions of pseudopodia.
culture
Culturing the amoeba from patient samples can also aid in the diagnosis of Naegleria fowleri. However, culturing Naegleria fowleri can be challenging due to its fastidious nature and the need for specialized media. One commonly used medium is non-nutrient agar seeded with bacteria, such as Escherichia coli. The patient sample, usually CSF, is inoculated onto the agar and incubated at temperatures ranging from 37 to 42 degrees Celsius. Naegleria fowleri grows rapidly and forms characteristic amoebic colonies within a few days. The colonies can be further confirmed through microscopic examination. Culturing provides an opportunity for future research and antimicrobial susceptibility testing.
Molecular Techniques:
Advances in molecular techniques have revolutionized the diagnosis of infectious diseases, including Naegleria fowleri infections. Polymerase chain reaction (PCR) is a sensitive and specific method that can detect the DNA of Naegleria fowleri in patient samples. Specific primers designed to target the DNA of Naegleria fowleri are used to amplify and detect its presence. PCR allows for rapid and accurate diagnosis, aiding in early intervention and potentially saving lives. A nested PCR assay has also been applied to detect the presence of the parasite in domestic water sources
serological methods
Immunodiagnostic methods have also been developed for the detection of Naegleria fowleri. Enzyme-linked immunosorbent assays (ELISAs) have been developed to detect specific antibodies against Naegleria fowleri in patient serum or CSF. These assays utilize specific antigens of the amoeba to capture and detect antibodies produced in response to the infection. Immunodiagnostic (serology) testing has no role in the diagnosis of acute PAM
Conclusion:
The laboratory diagnosis of Naegleria fowleri plays a critical role in identifying infections caused by this deadly amoeba. Microscopic examination, culturing techniques, and molecular methods approaches all contribute to the accurate and timely detection of Naegleria fowleri. Each method has its strengths and limitations, and often, a combination of techniques is employed to maximize diagnostic accuracy. Early diagnosis allows for prompt treatment and offers the best chance for positive patient outcomes in the face of this rare but severe infection.